COVID-19 Coronavirus UK and World News Update Friday 1st October 2021
(Some UK data is delayed today)
UK Daily Statistics:
Cases: 7,842,613 (+35,577)
Losses of Life: 136,789 (+127)
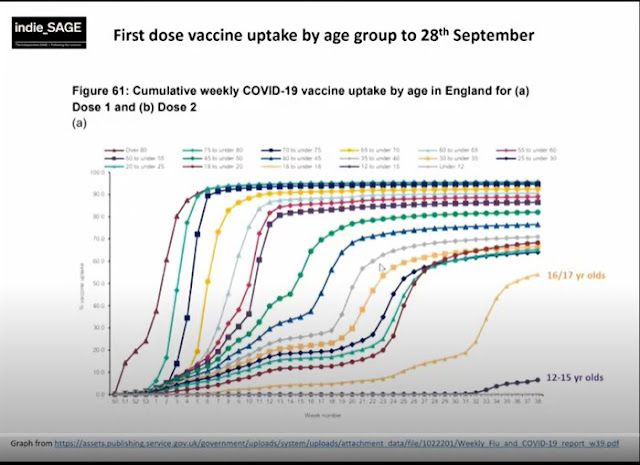
Vaccinations 1st Dose: 48,863,490
Vaccinations Fully Vaxxed: 44,901,832
Rep. Of Ireland: 390,989 (+1,057) cases and 5,249 losses of life.
World: 234,772,640 reported cases and 4,801,177 losses of life.
"The world missed the target set by WHO of vaccinating 10% of all people in all countries by the end of September.
Vaccinating the Earth equitably is a moral, epidemiological, and economic necessity. #VaccinEquity"
The World Health Organisation.
German pharmaceutical giant Merck & Co. have released interim data (the trial wasn't due to finish yet) from their antiviral pill study for Molnupiravir - and it's fabulous. It reduced hospitalisations and deaths by half in people who had mild to moderate illness (recently infected people, within 5 days of symptoms starting, when it could still go either way). This hasn't yet been peer-reviewed, but the world is all over it like a rash. If there are any flaws we'll soon know, but it's looking great so far.
Because the safety data is all there, and the efficacy findings are so positive, it starts to become unethical to give patients the placebo, and the trial doesn't need any more proof that it works.
"7.3% of patients who received molnupiravir were either hospitalized or died through Day 29 following randomization (28/385), compared with 14.1% of placebo-treated patients (53/377). Through Day 29, no deaths were reported in patients who received molnupiravir, as compared to 8 deaths in patients who received placebo. At the recommendation of an independent Data Monitoring Committee and in consultation with the U.S. Food and Drug Administration (FDA), recruitment into the study is being stopped early due to these positive results. Merck plans to submit an application for Emergency Use Authorization (EUA) to the U.S. FDA as soon as possible based on these findings and plans to submit marketing applications to other regulatory bodies worldwide."
At Day 29 the reduction in risk of death was looking far better than 50%, but some patients are still in hospital, so we can't yet say what the final outcome will be. (Hopefully positive all round - fingers crossed for them all.)
If Molnupiravir is approved, it will be the first oral antiviral treatment for COVID-19. Nice.